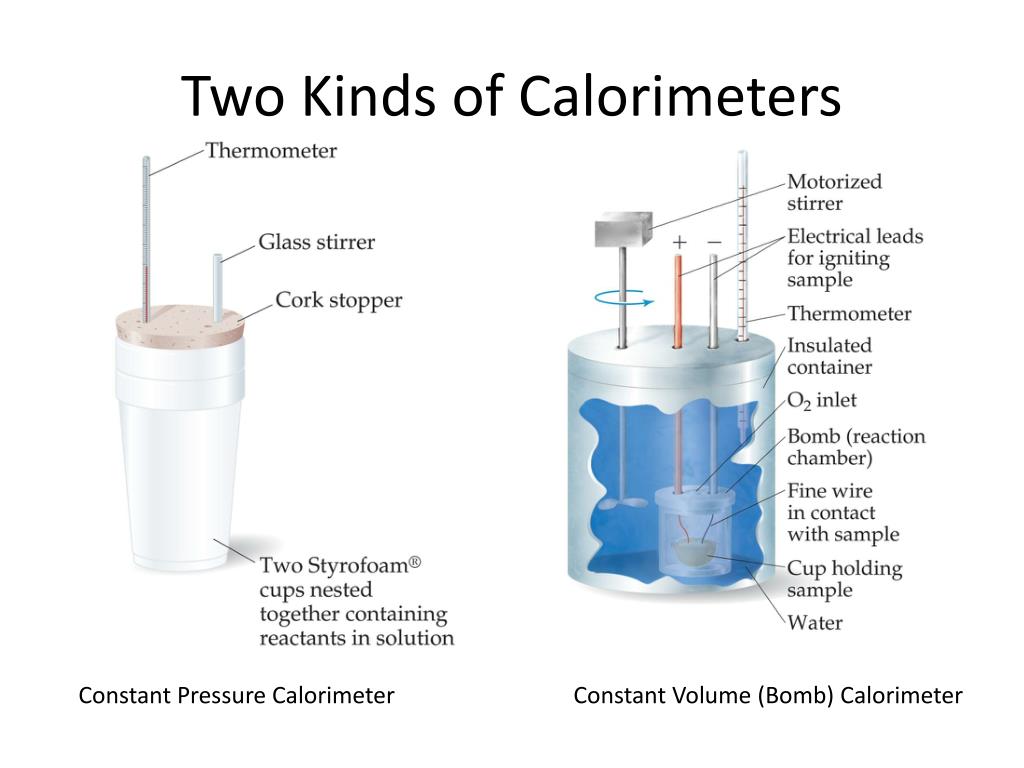
Calorimetry What Is The Importance . chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the. For example, when an exothermic reaction. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.slideserve.com
calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. From a chemical reaction like. what is calorimetry?
PPT Calorimetry PowerPoint Presentation, free download ID3850751
Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry What Is The Importance calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. From a chemical reaction like. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy. Calorimetry What Is The Importance.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry What Is The Importance what is calorimetry? chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. For example, when an exothermic reaction. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with. Calorimetry What Is The Importance.
From studylib.net
Calorimetry Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed. Calorimetry What Is The Importance.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry What Is The Importance It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure. Calorimetry What Is The Importance.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is calorimetry? chemists use calorimetry to determine the amount of heat transferred to or from a system into its. Calorimetry What Is The Importance.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry What Is The Importance calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we. Calorimetry What Is The Importance.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. For example, when an exothermic reaction. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. one technique we can use to measure the amount of heat involved. Calorimetry What Is The Importance.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry What Is The Importance For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It uses devices called calorimeters, which measure the. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction. Calorimetry What Is The Importance.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimetry What Is The Importance From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. what is calorimetry? For example, when an exothermic reaction. calorimetry is the set of techniques. Calorimetry What Is The Importance.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry What Is The Importance calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is calorimetry? a calorimeter is. Calorimetry What Is The Importance.
From studyfinder.org
How to Use a Calorimetry Gizmo to Get the Answers You Need Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. what is calorimetry? From a chemical reaction like. calorimetry is the set of techniques used to measure enthalpy changes during chemical. Calorimetry What Is The Importance.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry What Is The Importance a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. From a chemical reaction like. For example, when an exothermic reaction. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. calorimetry is the set of techniques used to measure. Calorimetry What Is The Importance.
From iqclasses.in
calorimetry chapter important notes class10 icse Calorimetry What Is The Importance calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is calorimetry? From a chemical reaction like. a calorimeter is a. Calorimetry What Is The Importance.
From users.highland.edu
Calorimetry Calorimetry What Is The Importance For example, when an exothermic reaction. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. It uses devices called calorimeters, which measure the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we can use to measure the. Calorimetry What Is The Importance.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry What Is The Importance Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the. what is calorimetry? From a chemical reaction like. a calorimeter is a device used to measure the. Calorimetry What Is The Importance.
From studylib.net
Chapter 5b (PowerPoint) Calorimetry What Is The Importance calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. It uses devices called calorimeters, which measure the. chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. what is calorimetry? Calorimetry is a branch of science. Calorimetry What Is The Importance.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry What Is The Importance chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. From a chemical reaction like. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical. Calorimetry What Is The Importance.
From exoxbgvei.blob.core.windows.net
Calorimeter Laboratory at Clara Carter blog Calorimetry What Is The Importance one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. what is calorimetry?. Calorimetry What Is The Importance.